
Immunohistochemistry (IHC) experiments involve a complex workflow, and each step from specimen preparation to final chromogenic development may affect the accuracy of experimental results. This article systematically summarizes the common problems, typical examples, and corresponding solutions in each key step of the experiment, providing references for researchers to avoid errors and obtain reliable results.
I. Problem Analysis in Specimen Preparation
Specimen preparation is the foundation of IHC experiments, including three core steps: fixation, tissue sampling/dehydration/paraffin infiltration, and sectioning/floating/baking. Improper operation in any step will lead to subsequent abnormal staining.
(I) Problems in Specimen Fixation
Common issues: Delayed or incomplete tissue fixation can result in gray and blurred cell nuclei with poor contrast in HE staining; unsatisfactory IHC results—typically, antigens are completely lost 2 hours after tissue excision.
1 Typical examples:
CD45 staining of paraffin-embedded human lymph node sections: Due to insufficient tissue fixation, lymphocytes at the edge of the lymph node show strong CD45 positivity, while those in the inner part of the tissue exhibit weak positivity, leading to uneven staining overall.
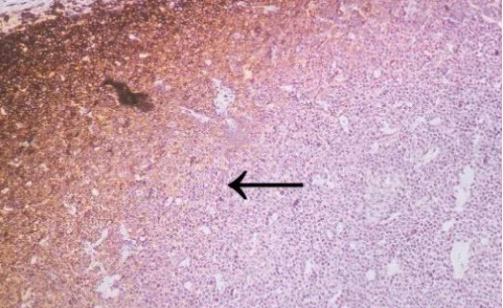
2 HE staining of paraffin-embedded renal tissue sections: Delayed or incomplete fixation results in blurred cell nuclei and poor contrast.
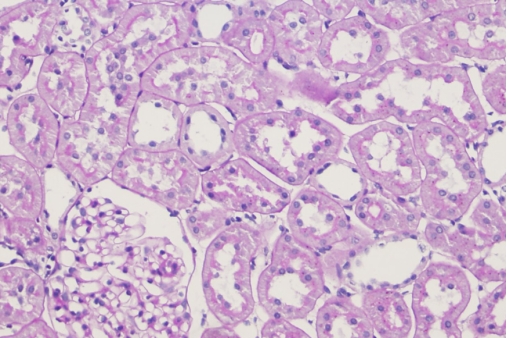
Recommendations for Resolution:
1 Tissues should be fixed as soon as possible after excision. The tissue block size should be controlled at 15×15×5 mm, and incising the tissue prior to fixation can enhance the fixation effect.
2 10% neutral buffered formalin is preferred as the fixative.
3 The fixation time should be limited to 4–24 hours; prolonged fixation may impair the exposure of antigenic determinants, potentially resulting in false-negative results.
4 The volume of the fixative should be at least 5 times that of the tissue to ensure adequate fixation.
(II) Problems in Tissue Sampling, Dehydration, and Paraffin Infiltration
Common issues: Inadequate tissue dehydration and paraffin infiltration can cause tissue shrinkage and depression in paraffin blocks, and increase the risk of section detachment during heat-induced antigen retrieval in IHC.
Recommendations for Resolution:
1 Gradient alcohol dehydration should be as thorough as possible to avoid residual moisture affecting subsequent paraffin infiltration.
2 The clearing time with xylene should not be excessively long; 1–3 hours is optimal. Over-clearing can lead to tissue hardening and brittleness.
3 Low-melting-point paraffin should be used for infiltration, and sufficient infiltration must be ensured to achieve perfect integration between the tissue and paraffin.
(III) Problems in Sectioning, Floating, and Baking
Common issues: Excessively thick sections, the presence of knife marks or wrinkles, and section detachment all interfere with result interpretation.
Typical example:
1 The presence of knife marks on sections directly interferes with the normal interpretation of results.
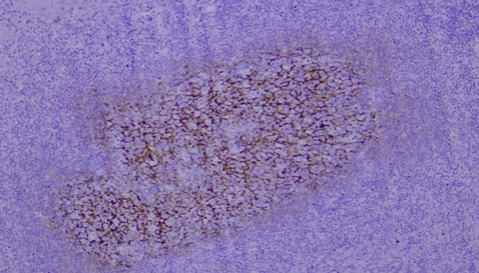
2 Staining of paraffin-embedded breast cancer tissue sections: Mammaglobin staining is positive, but section detachment occurs due to insufficient baking time.
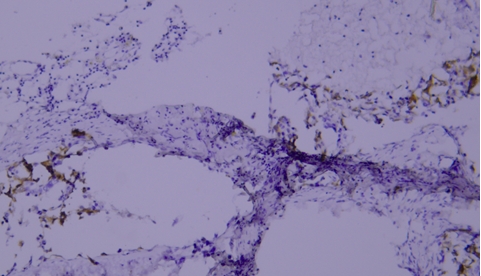
Recommendations for Resolution:
1 The section thickness should be adjusted according to the tissue type: lymph nodes, kidneys and other similar tissues need to be cut to no more than 3 μm; brain tissue (especially frozen sections of fresh specimens) can be slightly thicker; gastrointestinal, hepatobiliary and other tissues are routinely cut to 2–4 μm; the optimal thickness for conventional paraffin sections is 6–8 μm, and frozen sections can be cut to 10 μm. The sectioning angle and the freshness of the blade have a significant impact on section quality. The angle should be adjusted according to the microtome model, and new blades are preferred to obtain intact sections.
2 During floating, sections should be free of wrinkles and air bubbles, and the water temperature is optimally controlled at around 40°C.
3 For slide adhesion, protein glycerol (prepared by mixing egg white and glycerol at a 1:1 ratio) can be applied to the glass slides, or anti-detachment slides or polylysine-treated slides can be used to enhance the adhesion between the tissue and the slides.
4 The drying temperature is set at 50°C, and the time is controlled within 12–24 hours to ensure firm adhesion of the sections.
II. Problem Analysis in Staining Operation
The staining process includes dewaxing, antigen retrieval, blocking, washing, primary antibody incubation, secondary antibody incubation, DAB chromogenic development, hematoxylin counterstaining, etc. The detailed control of each step directly determines the staining quality.
(I) Incomplete Dewaxing
Common issues: Incomplete dewaxing can cause metachromasia in tissues, characterized by poor hematoxylin staining, non-selective HE staining, uneven staining, and in some cases, failure of eosin to stain.
Recommendations for Resolution: Adjust the dewaxing time flexibly according to room temperature. The core principle is to completely and thoroughly remove paraffin from the sections to avoid residual wax affecting subsequent staining.
(II) Antigen Retrieval Issues
Antigen retrieval is a critical step in IHC experiments. After formalin fixation and paraffin embedding, antigenic determinants are prone to cross-linking with nucleic acids, leading to changes in protein spatial structure and masking of antigenic determinants. This reduces the binding sites between antigens and antibodies, thereby decreasing the positive detection rate and staining intensity. High-temperature heating or protease hydrolysis can reverse this cross-linking reaction and restore the original conformation of proteins, which is known as antigen retrieval.
Common issues: Relative reduction in positive detection rate and staining intensity.
Key Influencing Factors and Analysis:
1 Impact of pH value: Staining intensity varies with different tissues and antigen retrieval solutions of different pH values.
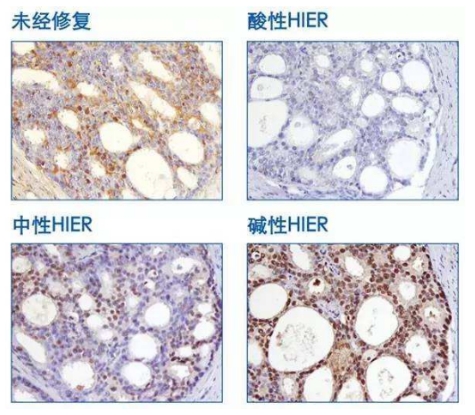
P27 staining of prostate tissue with antigen retrieval performed at 95°C for 10 minutes.
Practical Tips:
1 Common antigen retrieval buffers include citrate buffer (pH 6.0), EDTA buffer (pH 8.0–9.0), Tris/Tris-EDTA buffer (pH 9.0–10.0), and trypsin method (pH 3.5 ± 0.2).
2 The pH value of the retrieval buffer has a significant impact on staining results, and there is no universal antigen retrieval buffer.
3 Most antigens can achieve optimal retrieval effects in buffers with a pH range of 8.0–9.0; therefore, alkaline buffers are more widely used.
(III) Precautions for Blocking Step
1 The blocking agent should be selected based on whether the secondary antibody system contains biotin.
2 Blocking agents may be omitted for biotin-free secondary antibody systems.
3 For avidin-based secondary antibody systems, the corresponding blocking agent should be used to treat tissue sections prior to primary antibody incubation, depending on the type of biotin.
4 Excessively long blocking time can lead to weakened positive signals or even false negatives, while insufficient blocking time may result in increased background staining or even false positives. Strict control of blocking time is essential.
(IV) Inadequate Washing Issues
Typical example: CEA staining of paraffin-embedded human colon cancer tissue sections showed accumulation of staining solution (indicated by black arrows).
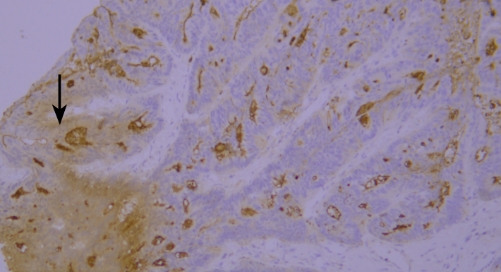
Recommendation for Resolution: Sufficient washing should be ensured during the experiment to avoid abnormal results caused by residual staining solution.
(V) Tissue Drying IssueTypical example:
TIMP-1 staining of paraffin-embedded human prostate tissue sections showed false negatives due to tissue drying (indicated by black arrows).
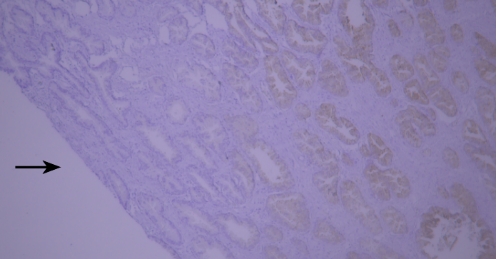
Recommendation for Resolution: Using a buffer supplemented with Tween-20 can effectively prevent section drying.
(VI) Edge Effect IssueTypical example:
Lysozyme staining of paraffin-embedded human tonsil tissue sections showed non-specific staining due to edge effect (indicated by black arrows).
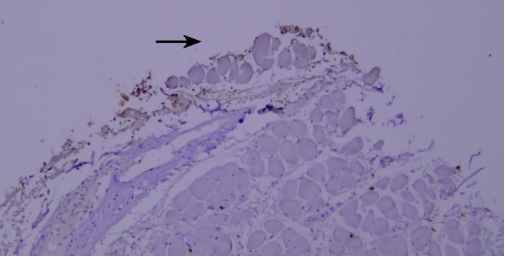
Recommendation for Resolution: Ensure firm adhesion between tissue sections and glass slides, and completely cover the tissue with reagents to prevent section drying; using a buffer supplemented with Tween-20 can reduce the occurrence of edge effect.
(VII) Issues Related to Primary Antibody Selection and Incubation
The selection of primary antibody is critical for achieving specific staining and reliable conclusions, and it should follow the principles of "high specificity, high sensitivity, low background, stable results, and good reproducibility."
Core Issues and Analysis:
1 Antibody Specificity: The specificity of an antibody is reflected in three aspects: tissue specificity, cellular specificity, and subcellular localization specificity.
Typical example: Staining of paraffin-embedded human prostate cancer tissue sections with different PSAP antibodies:
Left panel: Strong cytoplasmic positivity in cancer cells, indicating correct cellular specificity and subcellular localization of the antibody.
Right panel: Weak membranous positivity in cancer cells, where the antibody shows correct cellular specificity but incorrect subcellular localization, suggesting inaccurate specificity.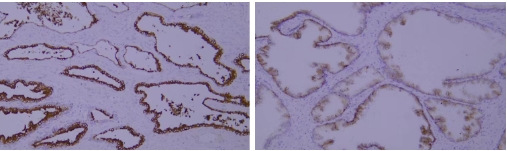
Recommendation for Resolution: Prioritize antibodies with accurate tissue specificity, cellular specificity, and subcellular localization specificity, as this is the core principle for primary antibody selection.
2 Antibody Staining Intensity Issue:
Typical example: Staining of paraffin-embedded human breast cancer tissue sections with different HER2 antibodies:
Left panel: Strong membranous positivity in cancer cells;
Right panel: Weak membranous positivity in cancer cells, indicating insufficient staining intensity of the antibody.
Common problem: Insufficient staining intensity can result in weak positivity in detection results.
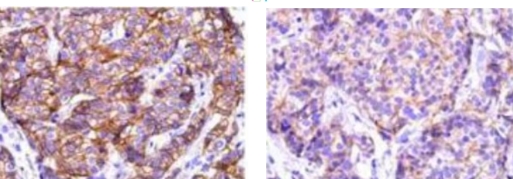
Recommendation for Resolution: Appropriately adjust the antibody dilution ratio, or select antibodies with high staining intensity and high affinity.
3 Issues with Primary Antibody Incubation Conditions:
Typical example: CEA staining of paraffin-embedded human colon cancer tissue sections:
When the primary antibody was incubated overnight at 4°C, strong cytoplasmic positivity in cancer cells was observed with a clean background;
When incubated at 37°C for 60 minutes, moderate cytoplasmic positivity in cancer cells was noted with a clean background.
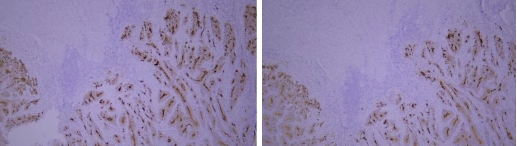
Conclusion: Incubation conditions have a slight impact on staining results, and it is necessary to screen for appropriate incubation conditions.
(VIII) Issues Related to Tissue-Specific Differences
Common problem: Staining results are inconsistent with expectations, showing negative or weak positive detection.
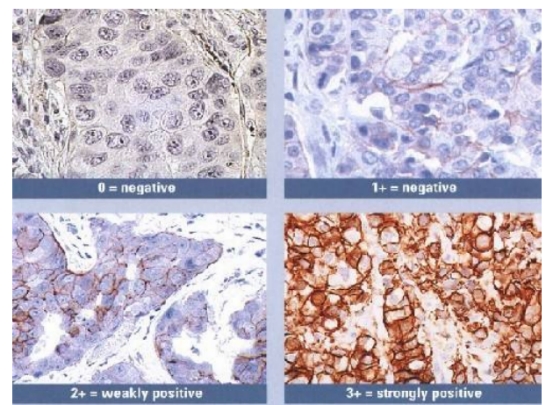
HER-2 staining results vary among different breast cancer samples.
Recommendation for Resolution: Set up positive tissue section controls. Due to significant differences in the expression of the same protein among different tissues, controls can help exclude the influence of the tissues themselves.
(IX) Issues Related to Secondary Antibody Selection
Core Conclusion: Different chromogenic systems exhibit distinct chromogenic effects. Polymer enzyme-labeled secondary antibodies offer higher sensitivity, cleaner background, and more distinct contrast compared to conventional HRP-directly labeled secondary antibodies.
Typical Example:CD34 staining of paraffin-embedded human placental tissue sections:
HRP-directly labeled secondary antibody: Weak positive staining with low background;
Polymer enzyme-labeled secondary antibody: Strong positive staining with clean background and distinct contrast.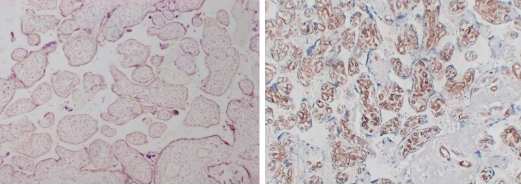
Conclusion: Different chromogenic systems yield distinct chromogenic results. Polymer enzyme-labeled secondary antibodies demonstrate higher sensitivity, cleaner background, and more distinct contrast compared to conventional HRP-directly labeled secondary antibodies.
(X) DAB Chromogenic Development Issues
1 DAB Flocculent or Aggregated Deposition:
Common problem: Dark brown to black flocculent or aggregated DAB deposits appear on tissue sections, interfering with result interpretation.
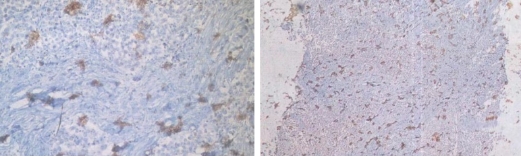
Recommendations for Resolution: ① DAB chromogenic solution should be freshly prepared and used immediately. Precipitation tends to form after preparation, so avoid using the solution that has been stored for an extended period. ② Flexibly adjust the chromogenic time. The time provided in the instruction manual is a range (30s–3min for fast-reacting solutions, 3–20min for slow-reacting solutions). After adding the solution, it is recommended to monitor and control the chromogenic time in real-time under a microscope.
2 Weak Signal:
Common problem: Insufficient intensity of the chromogenic signal affects the identification of positive results.
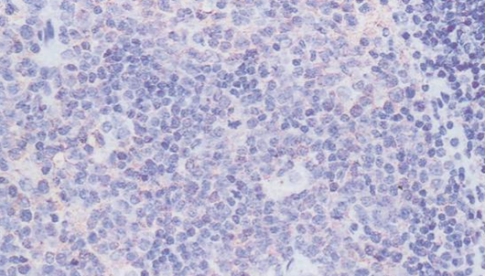
Recommendation for Resolution: Appropriately extend the chromogenic time. After adding the chromogenic solution, observe under a microscope and terminate the chromogenic reaction in a timely manner once the signal reaches the desired intensity.
(XI) Principles for Hematoxylin Use
1 Staining time varies among different hematoxylin formulations and is influenced by the freshness of the formulation.
2 Harris hematoxylin is commonly used internationally. Due to its mercury-containing formulation, differentiation and bluing steps are usually required, resulting in bright staining colors.
3 The modified Mayer's hematoxylin formulation does not require differentiation, and the staining time can be adjusted (15s–2min) based on the preparation time.
III. Summary
The accuracy of immunohistochemical experiments relies on refined operations at each step. From specimen preparation (fixation, dehydration, sectioning) to staining procedures (antigen retrieval, antibody selection, chromogenic development and counterstaining), each step must strictly follow operational standards. Meanwhile, parameters should be flexibly adjusted according to the characteristics of experimental tissues and antibodies. It is recommended that researchers fully understand the causes and solutions of various common problems before conducting experiments, set up appropriate positive and negative controls during the experiment, and promptly troubleshoot abnormalities to obtain reliable experimental results.
Related Promotional Journal Downloads

30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us



















