- Home
- Products
- Pathway
- Support
- Contact Us

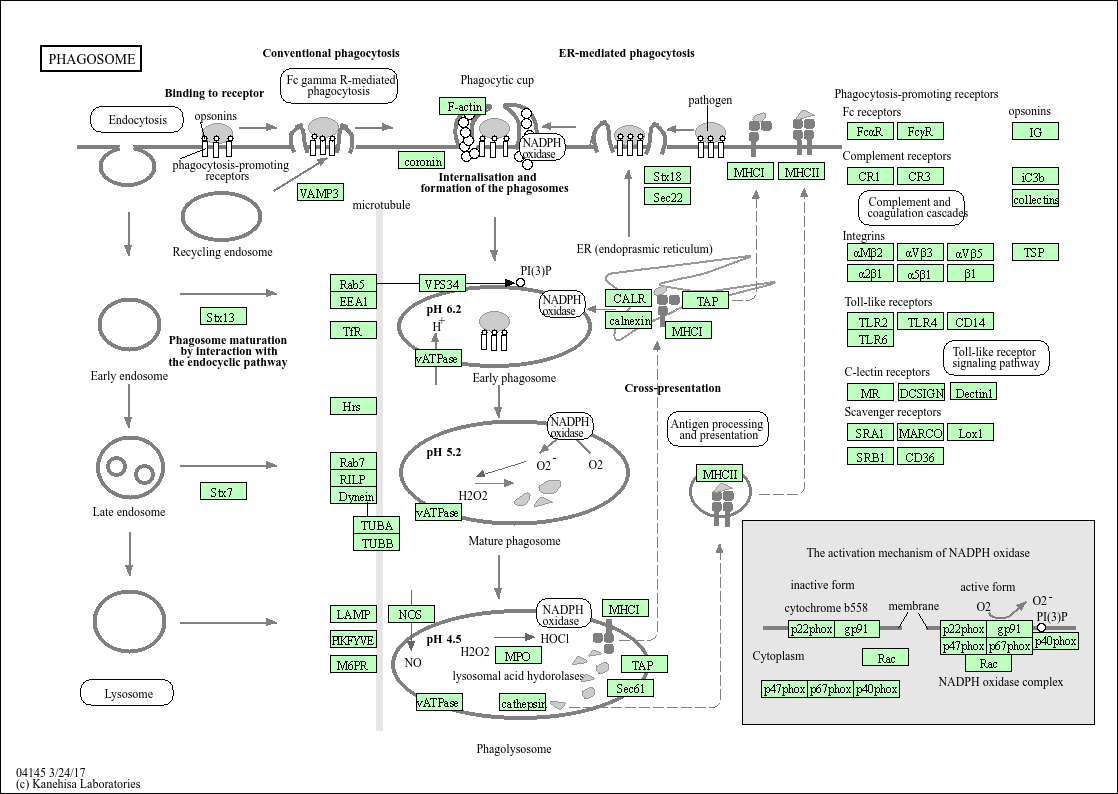
Phagosome
Core of basic research: Deciphers the molecular mechanism by which phagocytes (macrophages, neutrophils) internalize and degrade pathogens, apoptotic cells, or foreign bodies via phagocytosis, critical for innate immune defense and tissue homeostasis maintenance. The core process involves four steps: Phagocytes recognize targets via surface receptors (FcγR, CR3, TLR) and initiate phagocytic signals; signals trigger actin cytoskeleton rearrangement to form phagocytic cups encapsulating targets, which close to form phagosomes; phagosomes undergo acidification and maturation via sequential fusion with early and late endosomes; finally, phagosomes fuse with lysosomes to form phagolysosomes, degrading targets via acid hydrolases. During this process, TLR signals activate the NF-κB pathway to promote inflammatory factor secretion and enhance immune responses. Research focuses include the recognition specificity of phagocytic receptors, signal regulation of actin rearrangement, membrane transport mechanisms of phagosome maturation, regulation of phagosome-lysosome fusion, and the association of pathway abnormalities with infectious diseases (pathogen escape from phagocytic degradation) and autoimmune diseases (impaired apoptotic cell clearance).
Core key proteins: Phagocytic receptors (FcγRⅠ/Ⅱ/Ⅲ, CR3/CR4, TLR4/TLR2, scavenger receptors), Actin, actin regulatory proteins (Rac1, Cdc42, Arp2/3 complex), phagocytic signal kinases (Src, Syk, PI3K), Rab GTPases (Rab5/Rab7/Rab11, regulating phagosome maturation), phagosomal membrane proteins (LAMP1/LAMP2, participating in fusion), lysosomal hydrolases (cathepsins, nucleases), TLR signal adapters (MyD88, TRIF, activating inflammatory responses), NF-κB (transcriptional regulator of inflammatory factors).
Core key proteins: Phagocytic receptors (FcγRⅠ/Ⅱ/Ⅲ, CR3/CR4, TLR4/TLR2, scavenger receptors), Actin, actin regulatory proteins (Rac1, Cdc42, Arp2/3 complex), phagocytic signal kinases (Src, Syk, PI3K), Rab GTPases (Rab5/Rab7/Rab11, regulating phagosome maturation), phagosomal membrane proteins (LAMP1/LAMP2, participating in fusion), lysosomal hydrolases (cathepsins, nucleases), TLR signal adapters (MyD88, TRIF, activating inflammatory responses), NF-κB (transcriptional regulator of inflammatory factors).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















