- Home
- Products
- Pathway
- Support
- Contact Us

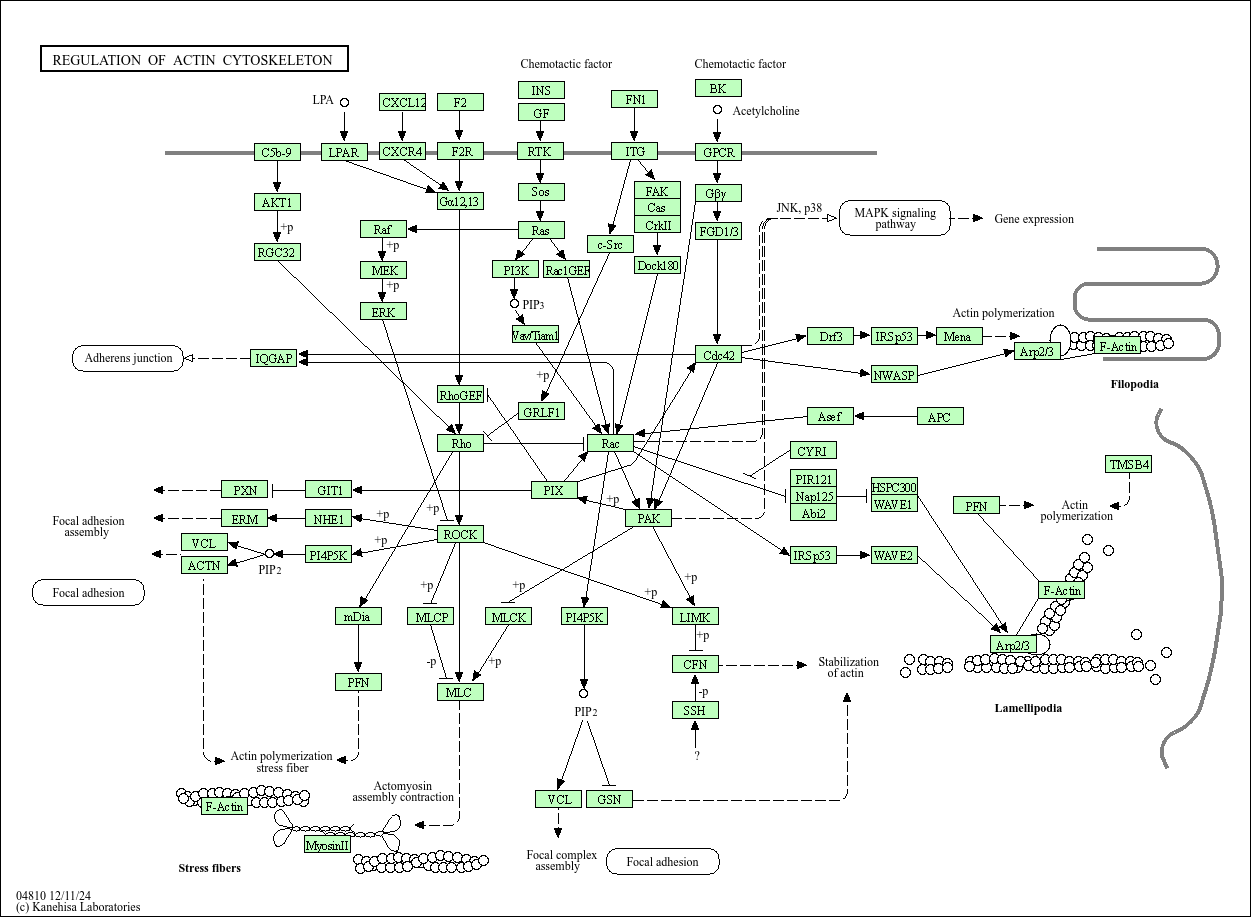
Regulation of actin cytoskeleton
Core of basic research: Deciphers the molecular mechanism of actin (Actin) assembly and remodeling, and its core role in cell shape maintenance, movement, division, and signal transduction. Actin monomers (G-actin) polymerize to form filamentous actin (F-actin), which assembles into functional structures such as parallel bundles, networks, or contractile rings under the regulation of various proteins. Core regulatory pathways include: The Rho GTPases family (Rac1, Cdc42, RhoA) regulating lamellipodia formation, filopodia extension, and stress fiber contraction respectively; the Arp2/3 complex promoting actin branched polymerization; the Formin family proteins promoting linear actin filament elongation; the Myosin family mediating relative sliding of actin filaments to generate contractile force. This pathway participates in physiological processes such as cell migration (e.g., cancer cell invasion), cell division (cytokinesis contractile ring), cell adhesion (focal adhesion-cytoskeleton connection), and phagocytosis (phagocytic cup formation). Research focuses include the dynamic balance of actin polymerization and depolymerization, the signal regulatory network of Rho GTPases, cooperation between actin and other cytoskeletons (microtubules, intermediate filaments), and the association of pathway abnormalities with tumor metastasis (enhanced cell migration) and cardiovascular diseases (abnormal vascular smooth muscle contraction).
Core key proteins: Actin (G-actin/F-actin), Rho GTPases family (Rac1, Cdc42, RhoA), Arp2/3 complex (promoting actin branching), Formin family (mDia1, FMNL), Myosin family (Myosin II, Myosin V), actin-binding proteins (Profilin, Cofilin, CapZ, Vinculin), focal adhesion-related proteins (FAK, Paxillin, connecting actin to the extracellular matrix), WASP/WAVE family (activating the Arp2/3 complex), LIM kinase (phosphorylating Cofilin to regulate actin depolymerization).
Core key proteins: Actin (G-actin/F-actin), Rho GTPases family (Rac1, Cdc42, RhoA), Arp2/3 complex (promoting actin branching), Formin family (mDia1, FMNL), Myosin family (Myosin II, Myosin V), actin-binding proteins (Profilin, Cofilin, CapZ, Vinculin), focal adhesion-related proteins (FAK, Paxillin, connecting actin to the extracellular matrix), WASP/WAVE family (activating the Arp2/3 complex), LIM kinase (phosphorylating Cofilin to regulate actin depolymerization).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















