- Home
- Products
- Pathway
- Support
- Contact Us

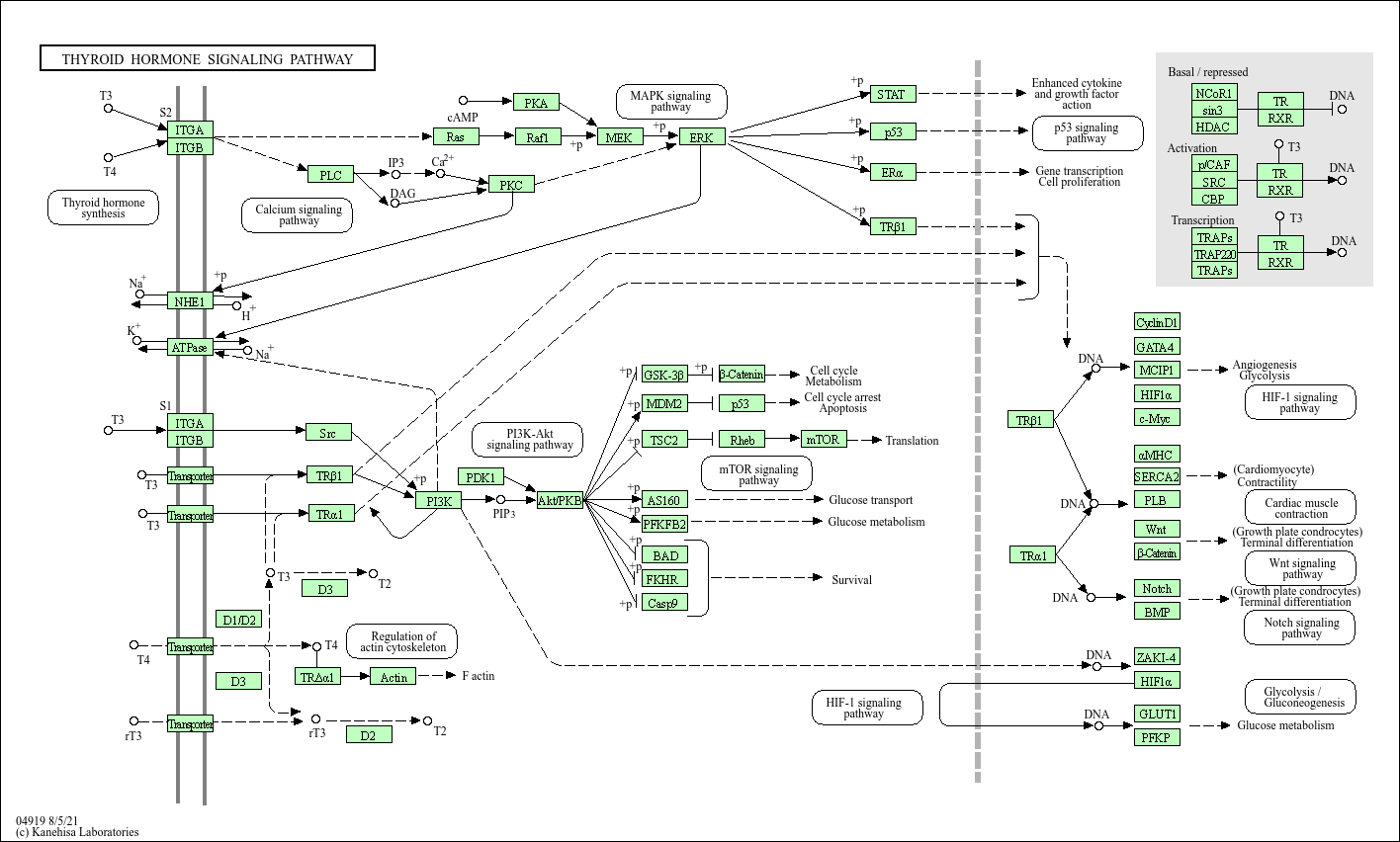
Thyroid hormone signaling pathway
Core of basic research: Clarifies the molecular mechanism by which thyroid hormones (T3, T4) regulate systemic metabolism, growth, development, and cell differentiation. Thyroid follicular epithelial cells synthesize and secrete T4 (tetraiodothyronine) and a small amount of T3 (triiodothyronine); T4 is converted to the active form T3 by deiodinases (D1/D2) in target cells. T3 binds to nuclear thyroid hormone receptors (TRα/TRβ), which form heterodimers with retinoic X receptors (RXR) and bind to thyroid hormone response elements (TRE) in target gene promoters to regulate transcription: in the liver and muscle, it activates fatty acid oxidation and glycolysis-related genes to increase energy consumption; in the nervous system and bones, it regulates cell proliferation and differentiation to ensure maturation. Additionally, thyroid hormones rapidly activate the PI3K-Akt and MAPK pathways via non-nuclear receptor pathways to regulate cell function. Research focuses on the regulation of T3 production and metabolism (D1/D2/D3 balance), TR transcriptional activation/inhibition mechanisms (binding coactivators p300 or corepressors NCoR), associations with thyroid dysfunction (hyperthyroidism, hypothyroidism), and molecular mechanisms of thyroid hormone resistance syndrome (TR mutations).
Core key proteins: Thyroid hormones (T3, T4), thyroid hormone receptors (TRα/TRβ), RXR (retinoic X receptor), deiodinases (D1/D2/D3), TRE (thyroid hormone response element), p300/CBP (transcriptional coactivators), NCoR/SMRT (transcriptional corepressors), NIS (sodium-iodide symporter), TPO (thyroid peroxidase, key thyroid hormone synthesis enzyme), TSHR (thyroid-stimulating hormone receptor), PI3K/Akt (non-nuclear receptor signal pathway), MAPK (ERK, non-nuclear receptor signal pathway).
Core key proteins: Thyroid hormones (T3, T4), thyroid hormone receptors (TRα/TRβ), RXR (retinoic X receptor), deiodinases (D1/D2/D3), TRE (thyroid hormone response element), p300/CBP (transcriptional coactivators), NCoR/SMRT (transcriptional corepressors), NIS (sodium-iodide symporter), TPO (thyroid peroxidase, key thyroid hormone synthesis enzyme), TSHR (thyroid-stimulating hormone receptor), PI3K/Akt (non-nuclear receptor signal pathway), MAPK (ERK, non-nuclear receptor signal pathway).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















