- Home
- Products
- Pathway
- Support
- Contact Us

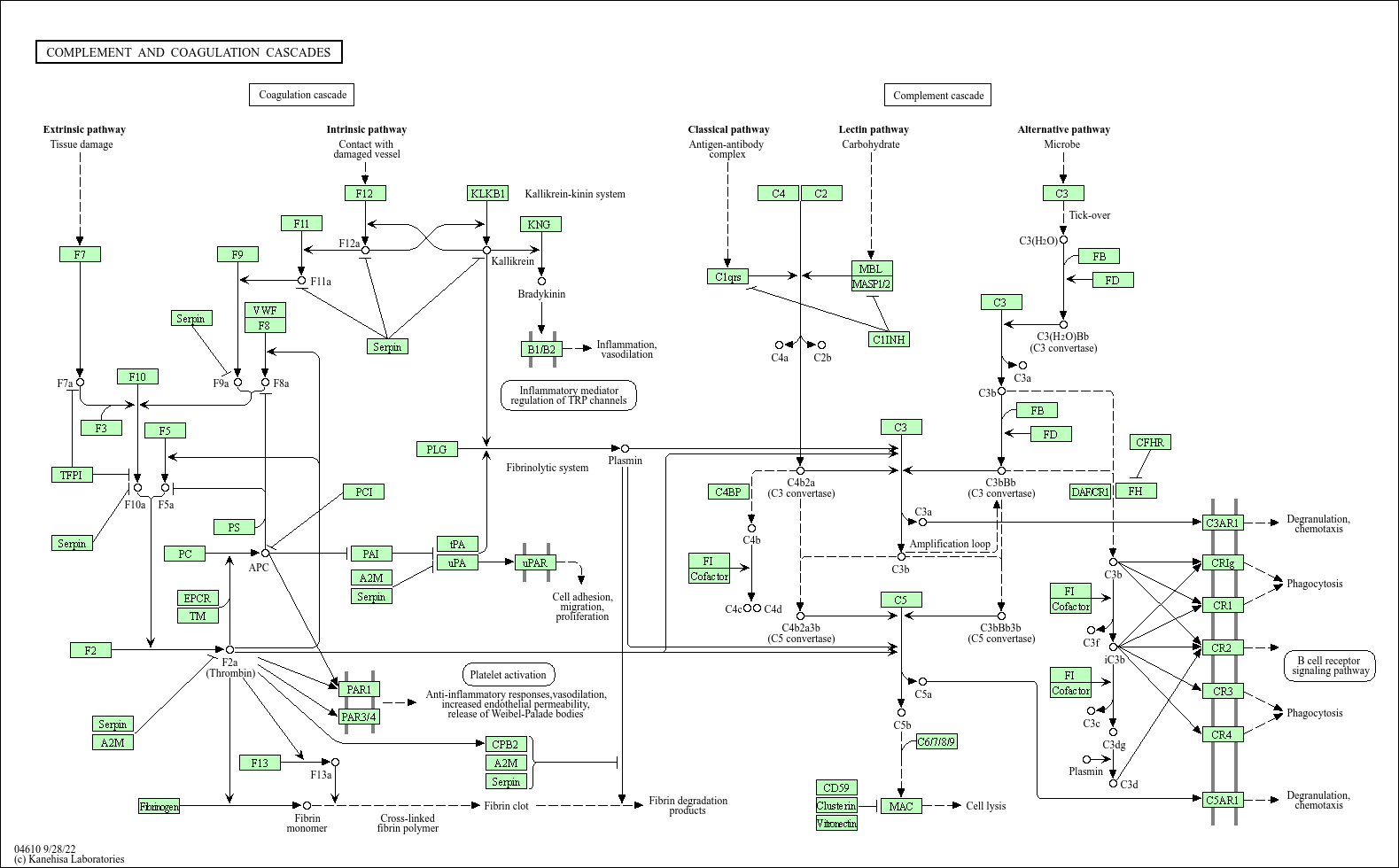
Complement and coagulation cascades
Core of basic research: Clarifies the cross-regulatory network of the complement and coagulation systems, key components of innate immunity that cooperate in inflammation, tissue repair, and pathological damage. The complement system is activated via the classical (C1q recognizing immune complexes), alternative (spontaneous C3 hydrolysis), and lectin (MBL recognizing pathogen glycans) pathways, generating C3 convertases (C4b2a, C3bBb) and C5 convertases, ultimately forming the membrane attack complex (MAC, C5b-9) for cell lysis, while releasing anaphylatoxins (e.g., C3a, C5a) to recruit immune cells. The coagulation system is activated via the extrinsic (TF activating FⅦ) or intrinsic (collagen activating FⅫ) pathway, with cascade activation of coagulation factors (FⅩ activation → thrombin generation) converting fibrinogen to fibrin for thrombus formation. Research focuses on cross-talk nodes (e.g., coagulation factor Xa activating complement C5; C3a promoting platelet activation), regulation of cascade activation thresholds (CD59 inhibiting MAC; antithrombin Ⅲ inhibiting thrombin), and tissue damage mechanisms from excessive activation in pathological states (sepsis, autoimmune diseases).
Core key proteins: Complement components (C1q, C3, C4, C5, C5b-9), complement receptors (CR1, CR3, C3aR, C5aR), coagulation factors (FⅡ, FⅤ, FⅦ, FⅩ, thrombin), TF (tissue factor), vWF (von Willebrand factor), PAI-1 (plasminogen activator inhibitor-1), CD59 (complement lysis inhibitor), CFH (complement factor H, negative regulator), fibrinogen, antithrombin Ⅲ (coagulation negative regulator), plasminogen (key fibrin degradation molecule).
Core key proteins: Complement components (C1q, C3, C4, C5, C5b-9), complement receptors (CR1, CR3, C3aR, C5aR), coagulation factors (FⅡ, FⅤ, FⅦ, FⅩ, thrombin), TF (tissue factor), vWF (von Willebrand factor), PAI-1 (plasminogen activator inhibitor-1), CD59 (complement lysis inhibitor), CFH (complement factor H, negative regulator), fibrinogen, antithrombin Ⅲ (coagulation negative regulator), plasminogen (key fibrin degradation molecule).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















