- Home
- Products
- Pathway
- Support
- Contact Us

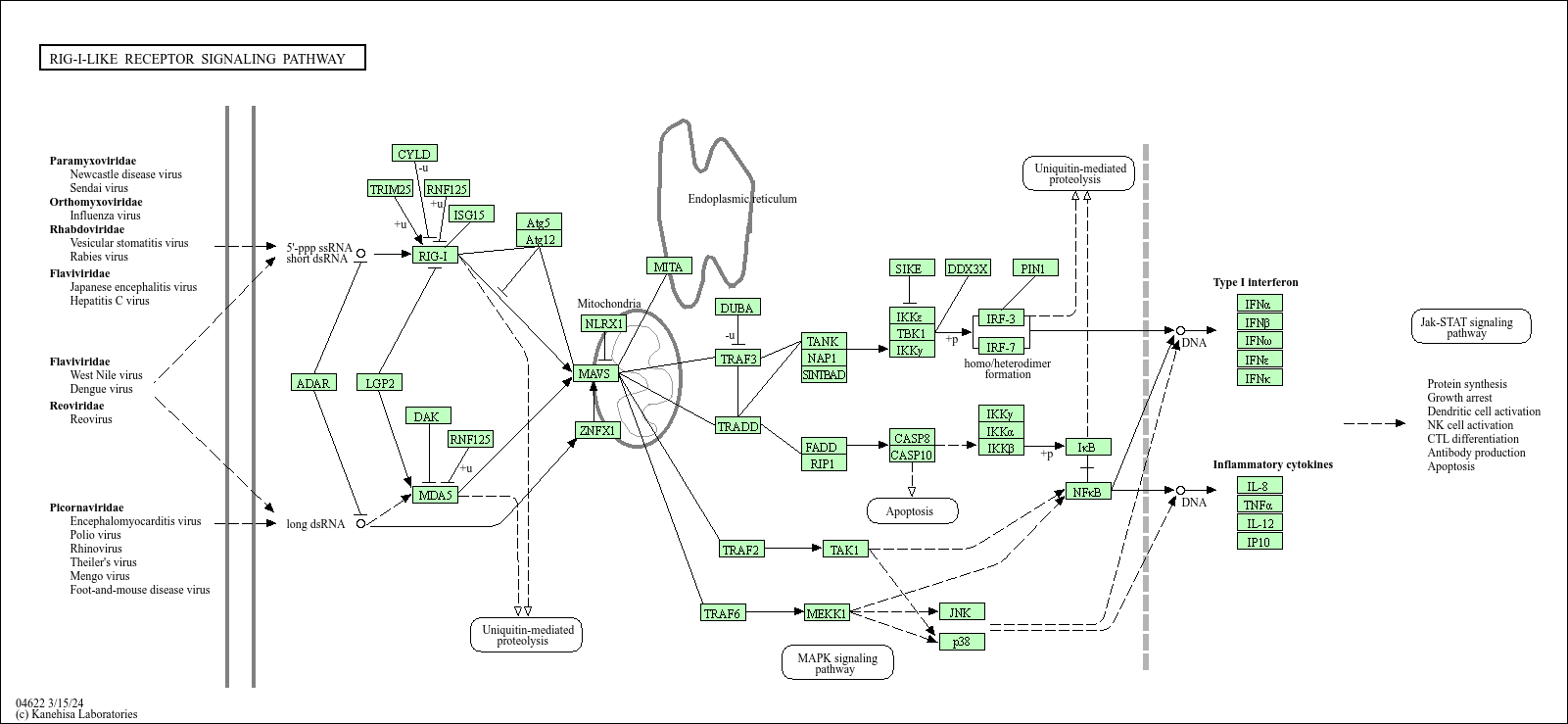
RIG-I-like receptor signaling pathway
Core of basic research: Focuses on the mechanism by which RIG-I-like receptors (RLR family) recognize cytoplasmic viral RNA to activate antiviral innate immunity, serving as the first line of defense against RNA viral infections. RIG-I recognizes viral RNA with 5' triphosphate groups, and MDA5 recognizes long double-stranded RNA. Both undergo conformational changes upon RNA binding, exposing CARD domains to interact with MAVS (IPS-1) on the mitochondrial membrane, inducing MAVS polymerization. Polymerized MAVS recruits TBK1 and IKK kinase complexes, which phosphorylate IRF3/7 and NF-κB respectively, initiating transcription of type I interferons (IFN-α/β) and inflammatory factors to establish an antiviral state. Research focuses on the mechanism of RLRs distinguishing viral from self-RNA, regulatory mechanisms of MAVS polymerization, roles of negative regulators (e.g., A20), and viral escape mechanisms (e.g., viral-encoded proteins degrading MAVS).
Core key proteins: RIG-I, MDA5 (RLR family members), MAVS (signal adaptor, also known as IPS-1), TBK1, IKKα/β, IRF3/7 (interferon regulatory factors), NF-κB, IFN-α/β, IFITM3 (antiviral effector protein), LGP2 (RLR family auxiliary protein), A20 (negative regulator).
Core key proteins: RIG-I, MDA5 (RLR family members), MAVS (signal adaptor, also known as IPS-1), TBK1, IKKα/β, IRF3/7 (interferon regulatory factors), NF-κB, IFN-α/β, IFITM3 (antiviral effector protein), LGP2 (RLR family auxiliary protein), A20 (negative regulator).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















