- Home
- Products
- Pathway
- Support
- Contact Us

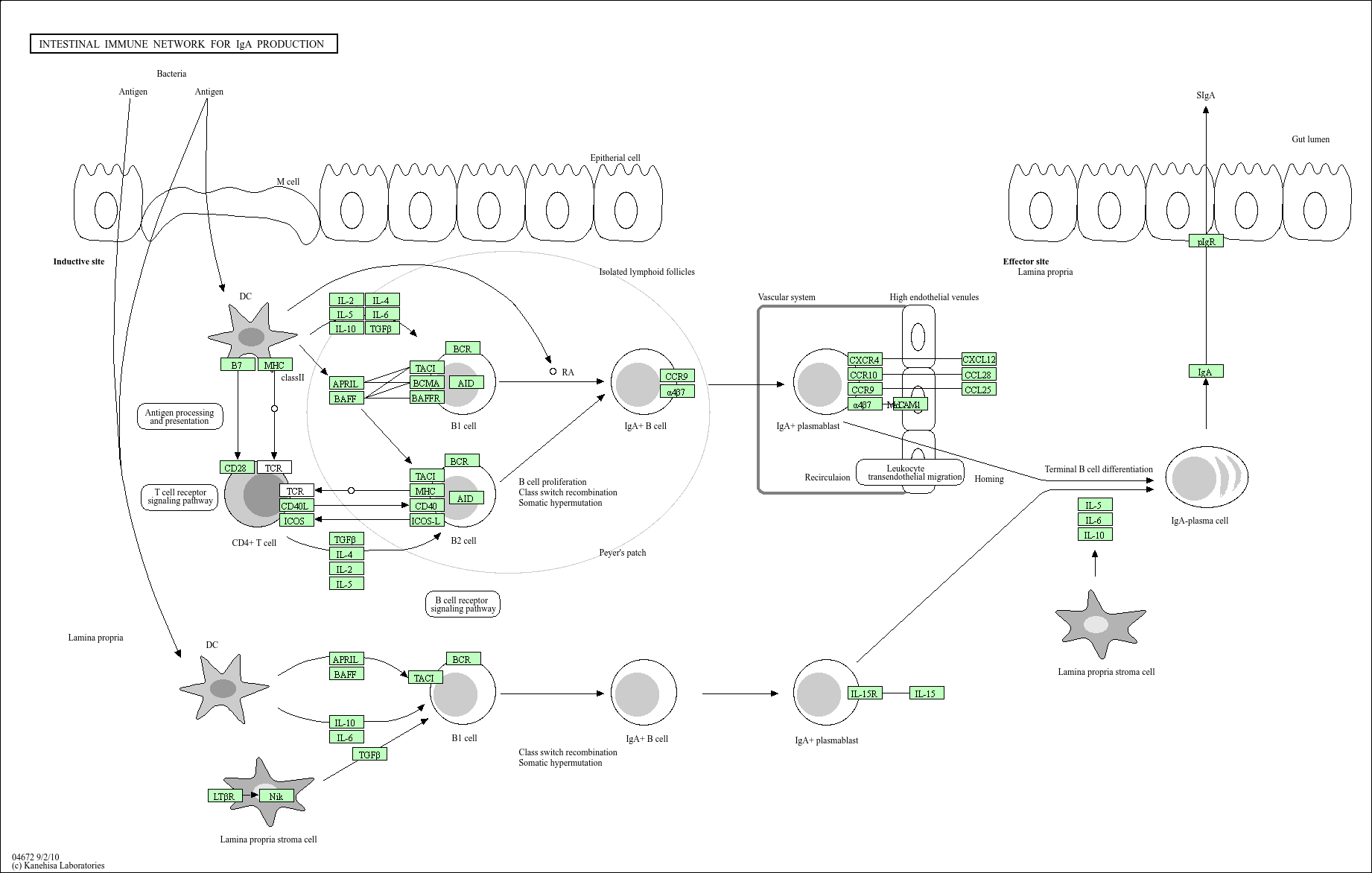
Intestinal immune network for IgA production
Core of basic research: As a core pathway of the intestinal mucosal immune barrier, it focuses on the regulatory mechanism of B cell differentiation into IgA-secreting plasma cells in the intestinal microenvironment. Naive B cells in the intestinal mucosa initiate IgA class switching (dependent on TGF-β) via T cell-dependent pathways (CD4+ T cells secrete IL-4/IL-5/IL-6, and CD40L binds to B cell CD40) or T cell-independent pathways (direct stimulation by intestinal flora metabolites) upon antigen stimulation. Differentiated plasma cells secrete dimeric IgA, which binds to Poly-IgR on the epithelial cell surface and is transported to the intestinal lumen to form secretory IgA (sIgA), playing roles in neutralizing pathogens and regulating intestinal flora balance. Research focuses include the regulatory mechanism of intestinal flora on IgA class switching, the inhibitory role of Treg cells in the pathway, interactions between mucosal epithelial cells and immune cells, and mechanisms of abnormal IgA secretion in intestinal inflammation (e.g., inflammatory bowel disease).
Core key proteins: IgA (monomer/dimer), TGF-β (IgA class switching inducer), IL-4/IL-5/IL-6 (B cell differentiation promoters), CD40/CD40L (costimulatory signals), J chain (IgA dimerization regulator), Poly-IgR (IgA transport receptor), intestinal flora metabolites (e.g., short-chain fatty acids), Treg cells (FOXP3+), BAFF (B cell survival factor).
Core key proteins: IgA (monomer/dimer), TGF-β (IgA class switching inducer), IL-4/IL-5/IL-6 (B cell differentiation promoters), CD40/CD40L (costimulatory signals), J chain (IgA dimerization regulator), Poly-IgR (IgA transport receptor), intestinal flora metabolites (e.g., short-chain fatty acids), Treg cells (FOXP3+), BAFF (B cell survival factor).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















