- Home
- Products
- Pathway
- Support
- Contact Us

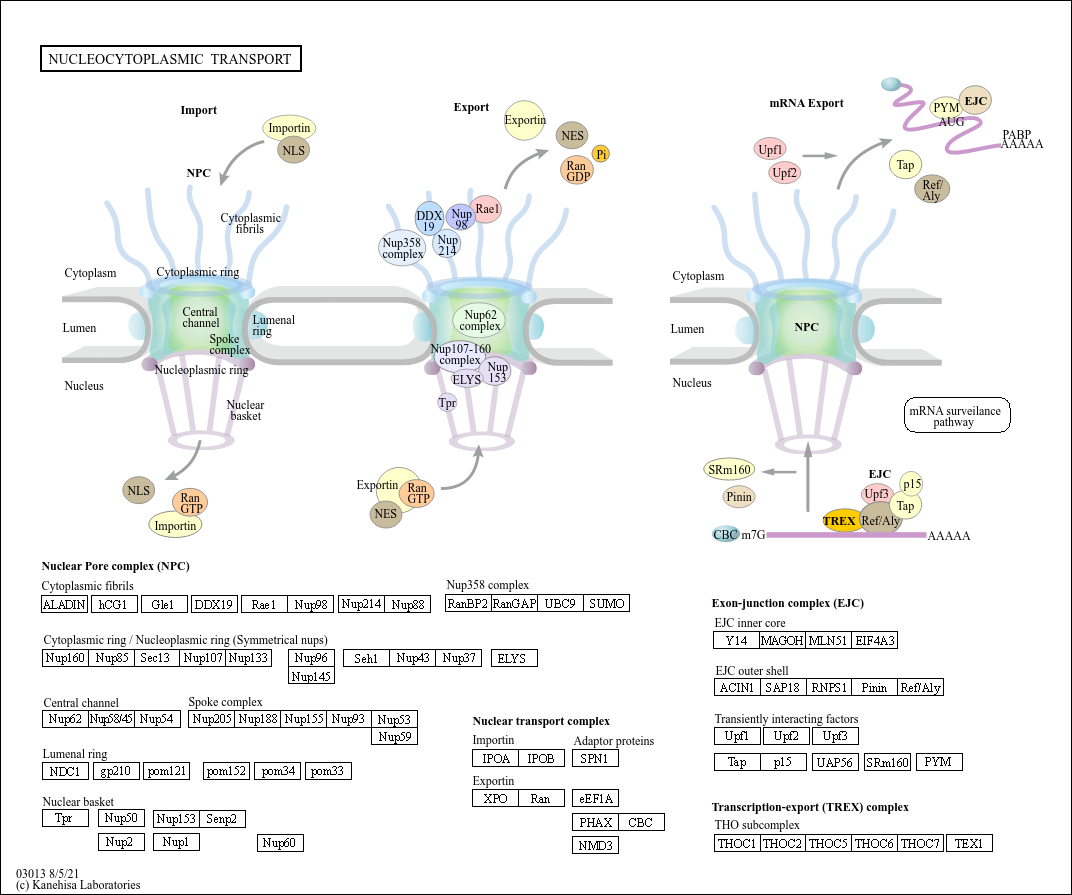
Nucleocytoplasmic transport
Core of basic research: Deciphers the molecular mechanisms of selective transport of macromolecules (proteins, RNA, ribosomal subunits) between the nucleus and cytoplasm, maintaining normal cellular physiological functions. The core regulatory system consists of nuclear pore complexes (NPC) and transport receptors: Proteins containing a nuclear localization signal (NLS) bind to import receptors (e.g., Importin α/β heterodimer) and enter the nucleus through NPC. In the nucleus, Ran-GTP binds to Importin β to dissociate the complex, releasing the target protein. Proteins containing a nuclear export signal (NES) bind to export receptors (e.g., Exportin 1/XPO1) and are exported to the cytoplasm through NPC with the assistance of Ran-GTP. The complex dissociates after Ran-GTP hydrolysis. Research focuses include the structure and function of NPC components, the regulation of Ran protein GTP/GDP cycle, the specificity of transport signal recognition, and the association of pathway abnormalities with tumors (e.g., overexpression of XPO1 leading to increased nuclear export of oncoproteins) and viral infections (viruses utilizing nucleocytoplasmic transport mechanisms to invade hosts).
Core key proteins: Nuclear pore complex (NPC) components (Nup153, Nup98, Nup62, POM121), Importin α/β (import receptors), Exportin 1/XPO1 (export receptor), Ran (small GTPase regulating transport direction), Ran-GAP (Ran GTPase-activating protein), Ran-GEF (Ran guanine nucleotide exchange factor), target proteins with NLS/NES signals (transcription factors, RNA-binding proteins), RNA transport proteins (e.g., TAP-p15 complex mediating mRNA export).
Core key proteins: Nuclear pore complex (NPC) components (Nup153, Nup98, Nup62, POM121), Importin α/β (import receptors), Exportin 1/XPO1 (export receptor), Ran (small GTPase regulating transport direction), Ran-GAP (Ran GTPase-activating protein), Ran-GEF (Ran guanine nucleotide exchange factor), target proteins with NLS/NES signals (transcription factors, RNA-binding proteins), RNA transport proteins (e.g., TAP-p15 complex mediating mRNA export).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















