- Home
- Products
- Pathway
- Support
- Contact Us

Lamarck Secondary Antibodies: Comprehensive Solutions for Research & Pathology, Balancing Breadth and Precision
As the critical bridge linking primary antibodies to detection signals in immunoassays like Western Blotting (WB), Immunohistochemistry (IHC), and Immunofluorescence (IF), secondary antibody performance directly impacts the accuracy, efficiency, and reliability of experimental results. Tailored to diverse application needs, Lamarck has built a secondary antibody portfolio covering both "general research use" and "pathology-specific IHC use" — meeting versatile research demands with a wide product range, while supporting clinical pathological testing with precise, high-efficiency performance. It provides full support for life science research and pathological diagnosis.
Pathological-Grade IHC Secondary Antibodies: Polymer Enzyme Conjugation Technology Empowers Accurate IHC Detection
Centered on polymer enzyme conjugation technology, pathological-grade IHC secondary antibodies are specifically designed for high-demand clinical pathological testing scenarios. They aim to address pain points of traditional secondary antibodies in signal amplification, background interference, and experimental efficiency through technical optimization.
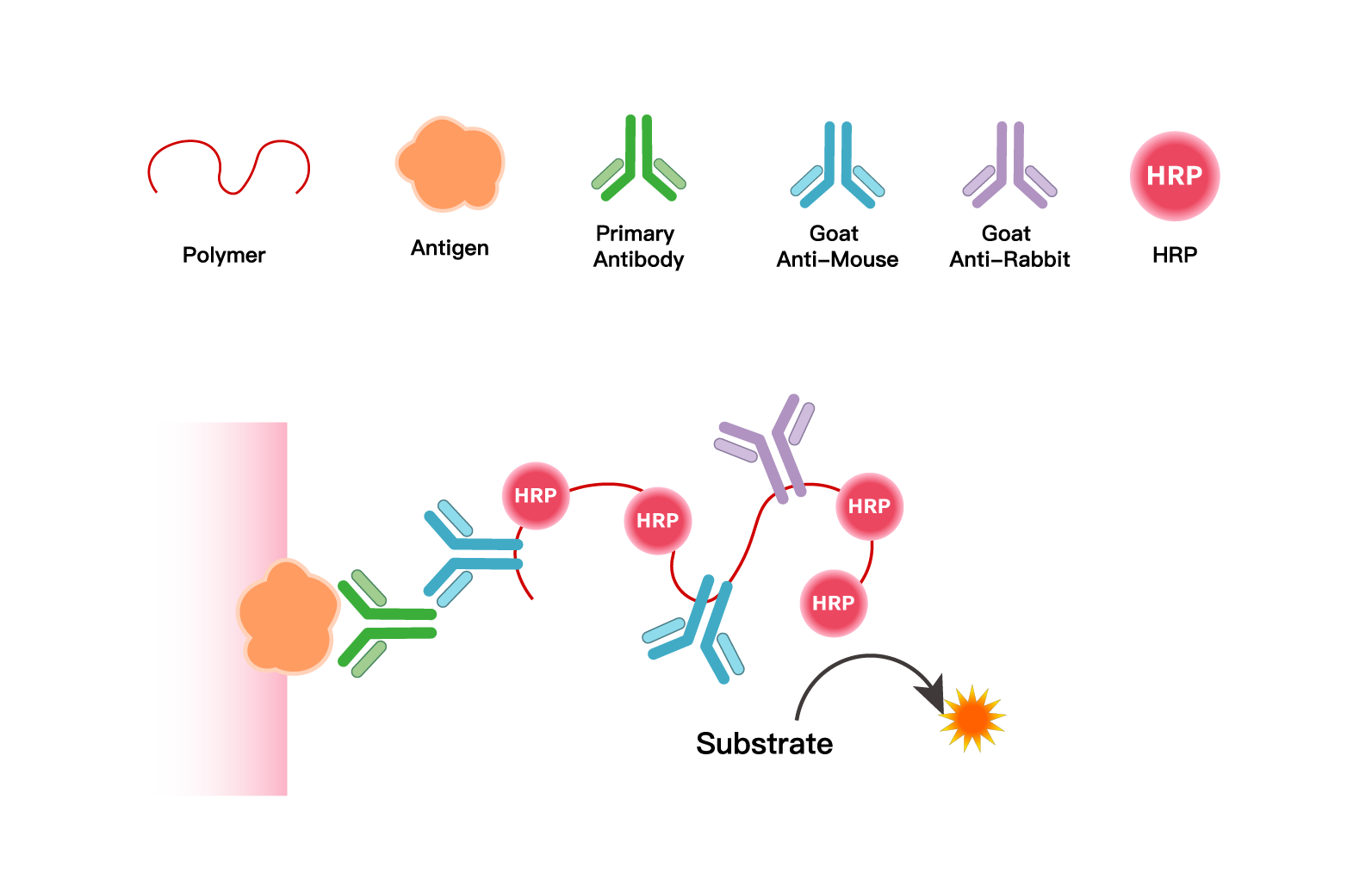
1. Core Technical Principle
Using polymers as carriers, their multi-site properties enable simultaneous conjugation of multiple peroxidase molecules and secondary antibody molecules targeting mouse/rabbit immunoglobulins on a single carrier. This creates an integrated IHC chromogenic system combining "signal amplification + specific binding," ultimately delivering precise, bright, and clear staining signals to ensure accurate localization and interpretation of target antigens in pathological sections.
2. Four Core Advantages, Aligned with Clinical Pathology Standards
Superior Core Performance: Adopting the polymer enzyme-conjugated two-step method, it offers high specificity, high sensitivity, and low background. The non-biotin conjugation design avoids endogenous interference, producing more accurate, clear, and bright detection results to guarantee signal quality.
Compliance with Clinical Standards: Validated through multiple indicators and tissue samples, it strictly adheres to clinical pathological diagnosis norms. Its performance is comparable to internationally renowned brands, meeting the high requirements of clinical testing for result reliability and consistency.
Broad Compatibility: Universal for mouse/rabbit species, eliminating the need to frequently replace secondary antibodies due to primary antibody species, and adapting to multi-scenario pathological testing needs.
Efficient & Convenient Operation: Ready-to-use design allows accurate detection completion with only 30 minutes of incubation, significantly simplifying procedures and improving experimental efficiency.
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















