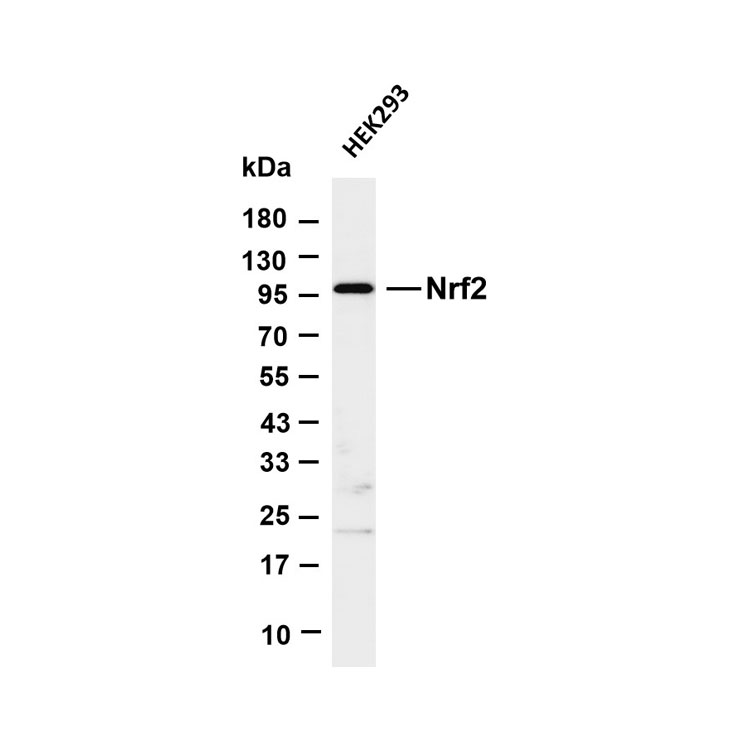
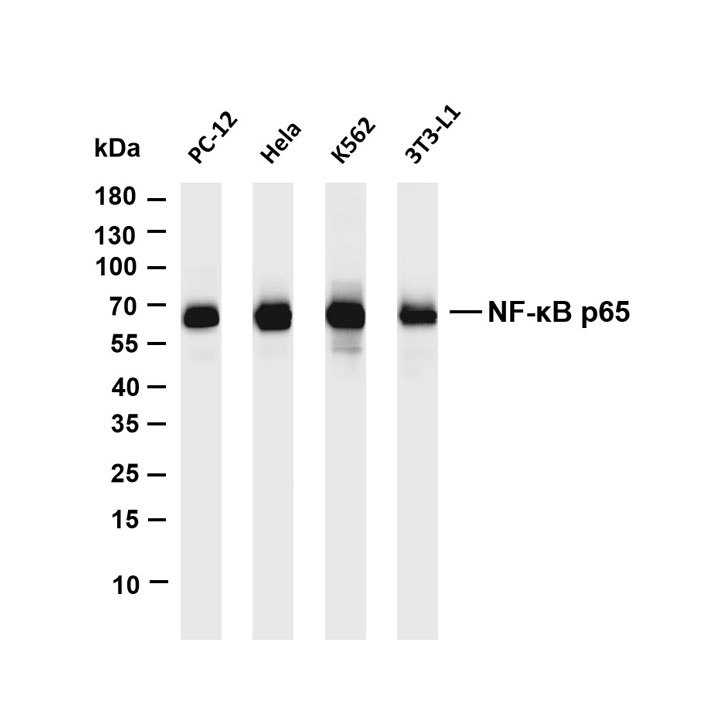
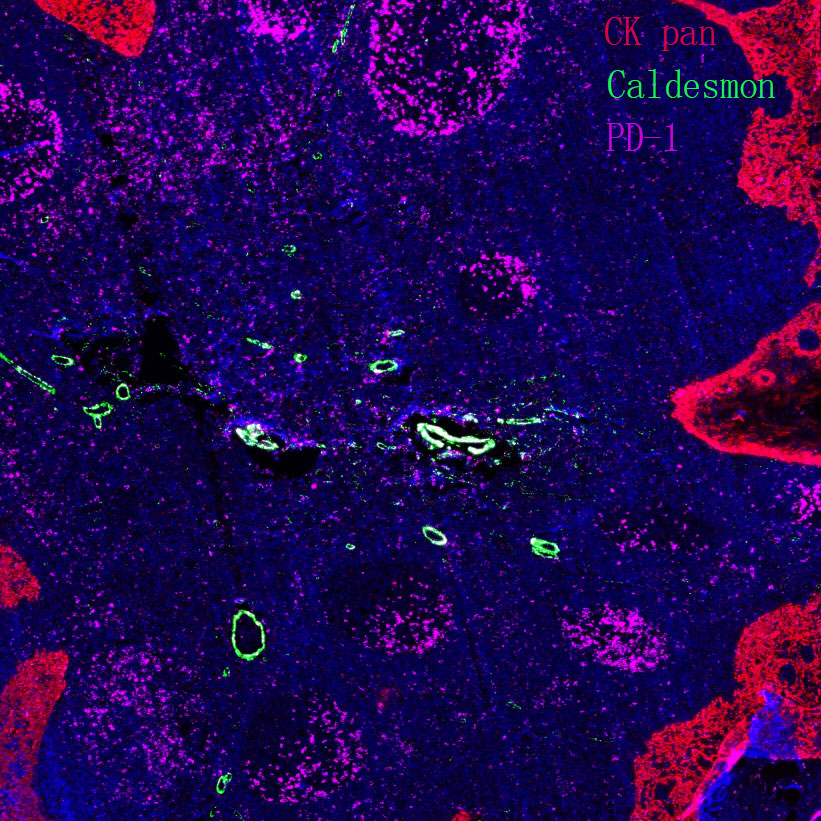
Pathological-Grade Primary Antibodies for IHC
Developed to meet the core goal of IHC experiments on human tissue samples, these antibodies cover the entire R&D process from immunogen design and positive cell line screening to antibody function validation. Guided by top international monoclonal antibody preparation experts throughout, they adhere to clinical testing standards and their performance is compared with that of international pathological antibody brands.
I. Precision R&D: Focused on IHC
1. Immunogen Design: Core Guarantee for Specificity
The selection and design of immunogens are critical to the success of antibody R&D. Synthetic peptide fragments are preferred as immunogens, under the guidance of top international monoclonal antibody experts. This ensures clear antigen epitopes, enhances antibody specificity and targeting from the source, and reduces non-specific binding interference in IHC experiments.
2. Cell Line Screening: Targeting High-Efficiency Secretory Cells
Guided by IHC experiment requirements, positive mice and B cells that secrete the target antibody are accurately screened via IHC detection on human tissue samples. On this basis, hybridoma cell lines are cultured and screened step-by-step to obtain high-quality cell lines that stably and efficiently secrete the target antibody, laying a foundation for subsequent antibody preparation.
3. Antibody Preparation and Preliminary Testing: Balancing Purity and Activity
The screened high-quality hybridoma cell lines are cultured in mouse peritoneal cavities, and high-purity monoclonal antibodies are obtained through ascites purification technology. Simultaneously, antibody purity testing, isotype identification, and IHC detection are completed.
II. Clinical-Grade Quality Control: Aligned with Top International Standards
Comprehensive Quality Control, Aligned with Clinical Standards
A verification system is established based on the strict requirements of clinical pathological testing. It not only conducts multi-dimensional checks on core indicators of purified antibodies (such as purity, isotype, and IHC performance) to ensure stable and uniform antibody performance, but also verifies via parallel control experiments with well-known international pathological antibody brands. Through horizontal comparison of IHC results on human tissue samples, the detection effect is confirmed to be consistent with and even superior to top international standards.
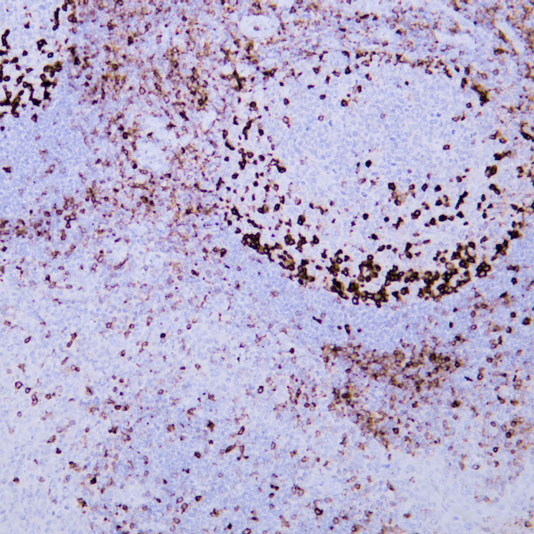
Target: CEA
Tissue: human rectal cancer
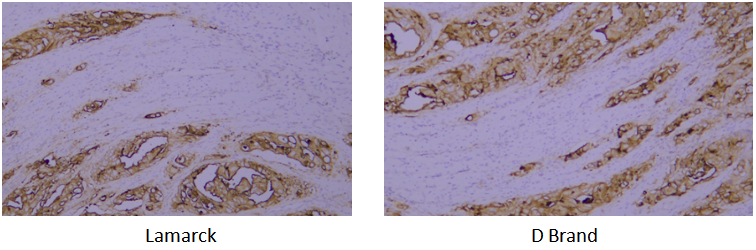
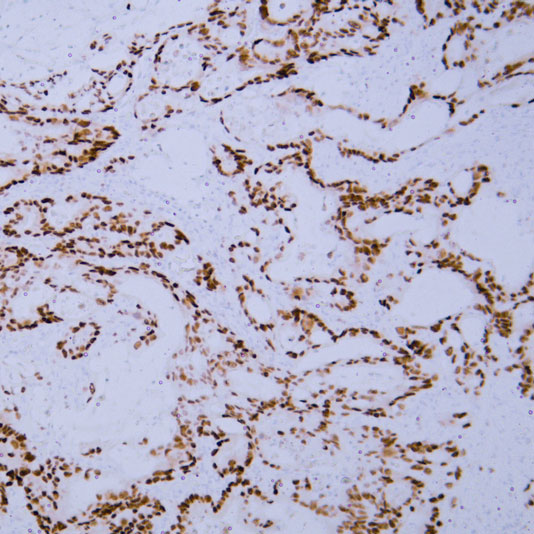
Target: CK-pan
Tissue: human oral squamous cell carcinoma
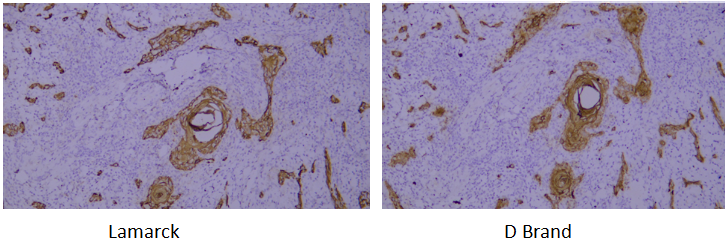
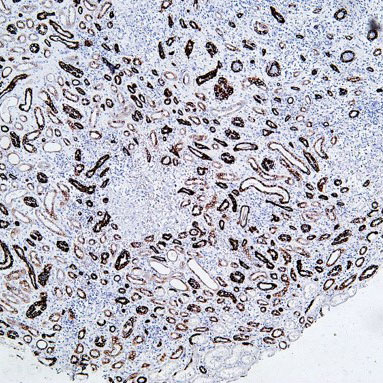
Target: CD31
Tissue: human liver
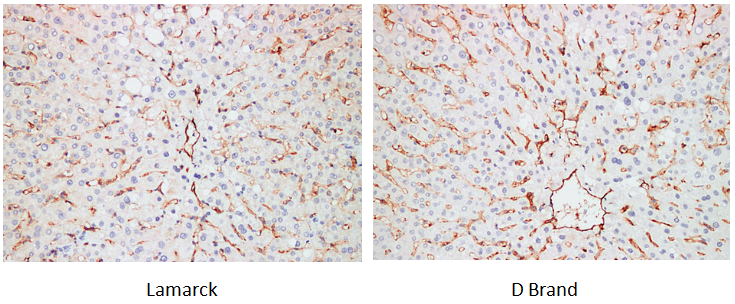
Outstanding High Specificity, Ensuring Accurate Diagnosis
The antibodies exhibit extremely high specificity in recognizing target antigens, enabling precise binding to specific proteins and antigen epitopes in tissues/cells. They rarely cause non-target cross-reactions, effectively avoiding "false positives" and "false negatives", and are key to distinguishing normal/lesioned tissues and clarifying pathological classification.
Adaptable to Complex Samples, Compatible with Mainstream Technologies
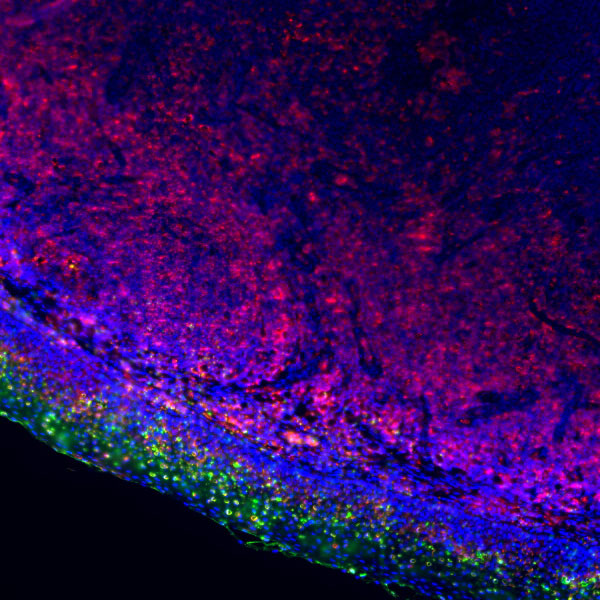
Addressing challenges in pathological experiments (such as diverse sample types including FFPE, frozen tissues, and cell smears, difficult antigen retrieval, and sample degradation), the antibodies are specially designed and verified to withstand pretreatment processes like high-temperature antigen retrieval and dewaxing/deparaffinization. They can effectively bind to targets even in samples with low antigen expression, and are compatible with mainstream pathological detection technologies such as IHC and IF.
Strong Batch Stability, Reproducible Results
Strict batch quality control (including titer detection, specificity verification, and stability testing) is implemented during production. Differences in titer and binding capacity between different antibody batches are minimal, ensuring consistent detection results across different times and operators. This meets the "standardized and reproducible" requirements of pathological experiments and avoids diagnostic deviations caused by batch fluctuations.
Verified in Clinical Scenarios, Improving Experimental Efficiency
Verified with clinical samples (e.g., comparison with tissue sections of known pathological results), the antibodies provide clear pathological application data (such as positive expression patterns in diseased tissues and recommended dilution ratios), improving experimental efficiency.
Pathological-Grade Secondary Antibodies for IHC
Centered on polymer enzyme conjugation technology, pathological-grade IHC secondary antibodies are specifically designed for high-demand clinical pathological testing scenarios. They aim to address pain points of traditional secondary antibodies in signal amplification, background interference, and experimental efficiency through technical optimization.
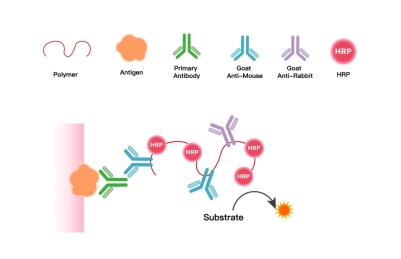
1. Core Technical Principle
Using polymers as carriers, their multi-site properties enable simultaneous conjugation of multiple peroxidase molecules and secondary antibody molecules targeting mouse/rabbit immunoglobulins on a single carrier. This creates an integrated IHC chromogenic system combining "signal amplification + specific binding," ultimately delivering precise, bright, and clear staining signals to ensure accurate localization and interpretation of target antigens in pathological sections.
2. Four Core Advantages, Aligned with Clinical Pathology Standards
Superior Core Performance: Adopting the polymer enzyme-conjugated two-step method, it offers high specificity, high sensitivity, and low background. The non-biotin conjugation design avoids endogenous interference, producing more accurate, clear, and bright detection results to guarantee signal quality.
Compliance with Clinical Standards: Validated through multiple indicators and tissue samples, it strictly adheres to clinical pathological diagnosis norms. Its performance is comparable to internationally renowned brands, meeting the high requirements of clinical testing for result reliability and consistency.
Broad Compatibility: Universal for mouse/rabbit species, eliminating the need to frequently replace secondary antibodies due to primary antibody species, and adapting to multi-scenario pathological testing needs.
Efficient & Convenient Operation: Ready-to-use design allows accurate detection completion with only 30 minutes of incubation, significantly simplifying procedures and improving experimental efficiency.
Related Promotional Journal Downloads

30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us