- Home
- Products
- Pathway
- Support
- Contact Us

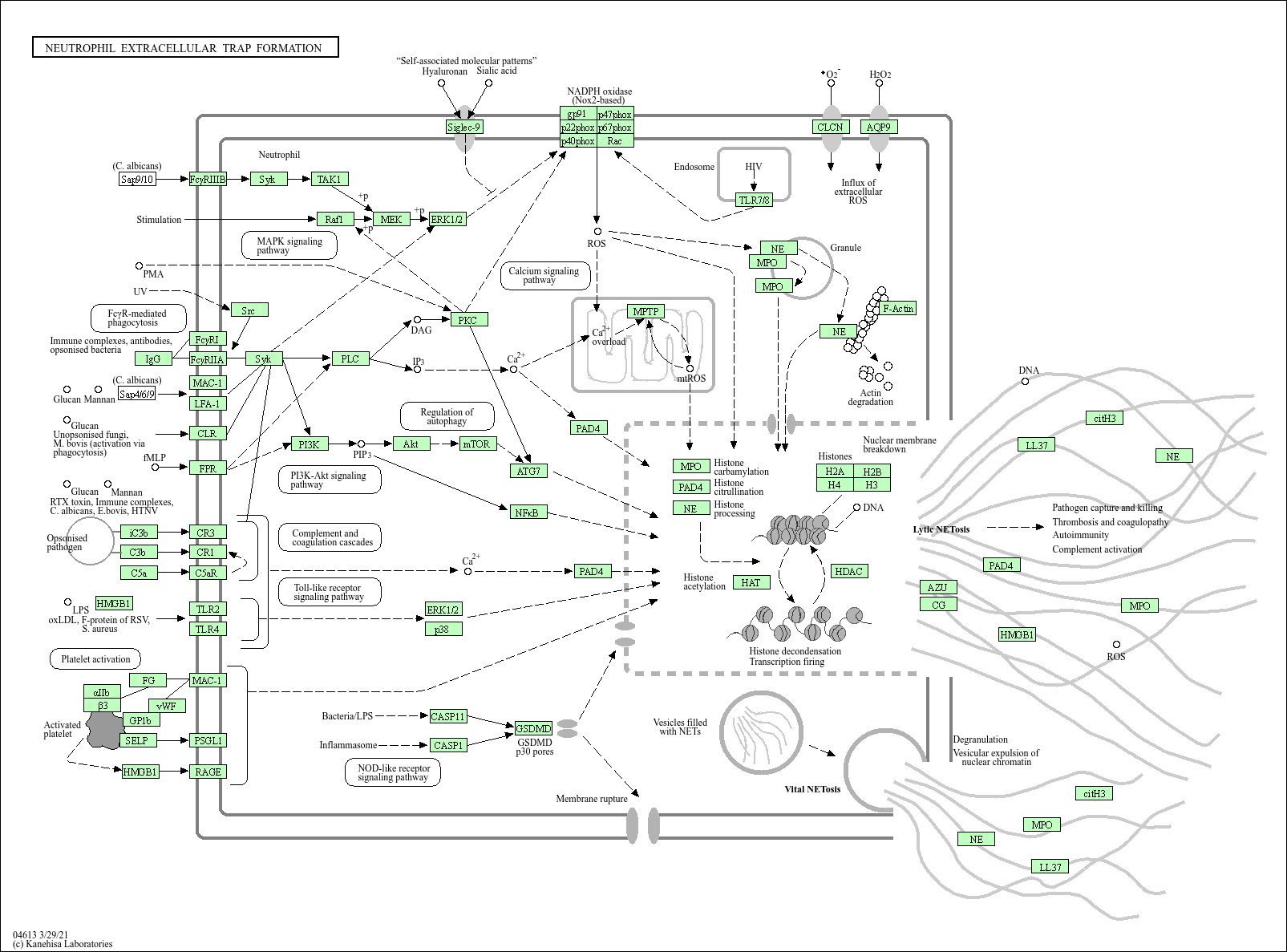
Neutrophil extracellular trap formation
Core of basic research: It focuses on the mechanism by which neutrophils release neutrophil extracellular traps (NETs) to capture and kill pathogens upon infection or inflammatory stimulation, an important anti-infective method of neutrophils. NETs consist of decondensed chromatin DNA, histones, and granular proteins (e.g., myeloperoxidase, elastase). After neutrophils are activated by stimulating signals (e.g., bacteria, cytokines, immune complexes), ROS production increases, activating PEPAD4 to citrullinate histones, leading to chromatin decondensation and extracellular release to form a network structure. NETs can capture pathogens such as bacteria, fungi, and viruses, and kill them through the enzymatic activity of granular proteins and the cationic properties of DNA. However, excessive NET formation can also damage surrounding tissues. Research focuses include the signal pathways of NET formation (e.g., PI3K-Akt, MAPK), regulation of histone citrullination, association between NETs and thrombosis, and mechanisms of excessive NET accumulation in pathological states (e.g., sepsis, autoimmune diseases).
Core key proteins: Neutrophils, DNA (chromatin), histones (H1, H2A, H2B, H3, H4), PEPAD4 (histone citrullination enzyme), myeloperoxidase (MPO), elastase (NE), ROS (reactive oxygen species), PI3K, Akt, MAPK (p38/ERK), TNF-α/IL-8 (activating signals), MMP9 (assists NET release).
Core key proteins: Neutrophils, DNA (chromatin), histones (H1, H2A, H2B, H3, H4), PEPAD4 (histone citrullination enzyme), myeloperoxidase (MPO), elastase (NE), ROS (reactive oxygen species), PI3K, Akt, MAPK (p38/ERK), TNF-α/IL-8 (activating signals), MMP9 (assists NET release).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















